
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
Original Article
- Drug/Regimen
- Safety and Effectiveness of Dulaglutide in the Treatment of Type 2 Diabetes Mellitus: A Korean Real-World Post-Marketing Study
- Jeonghee Han, Woo Je Lee, Kyu Yeon Hur, Jae Hyoung Cho, Byung Wan Lee, Cheol-Young Park
- Received February 3, 2023 Accepted July 10, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0030 [Epub ahead of print]
- 667 View
- 56 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader - Background
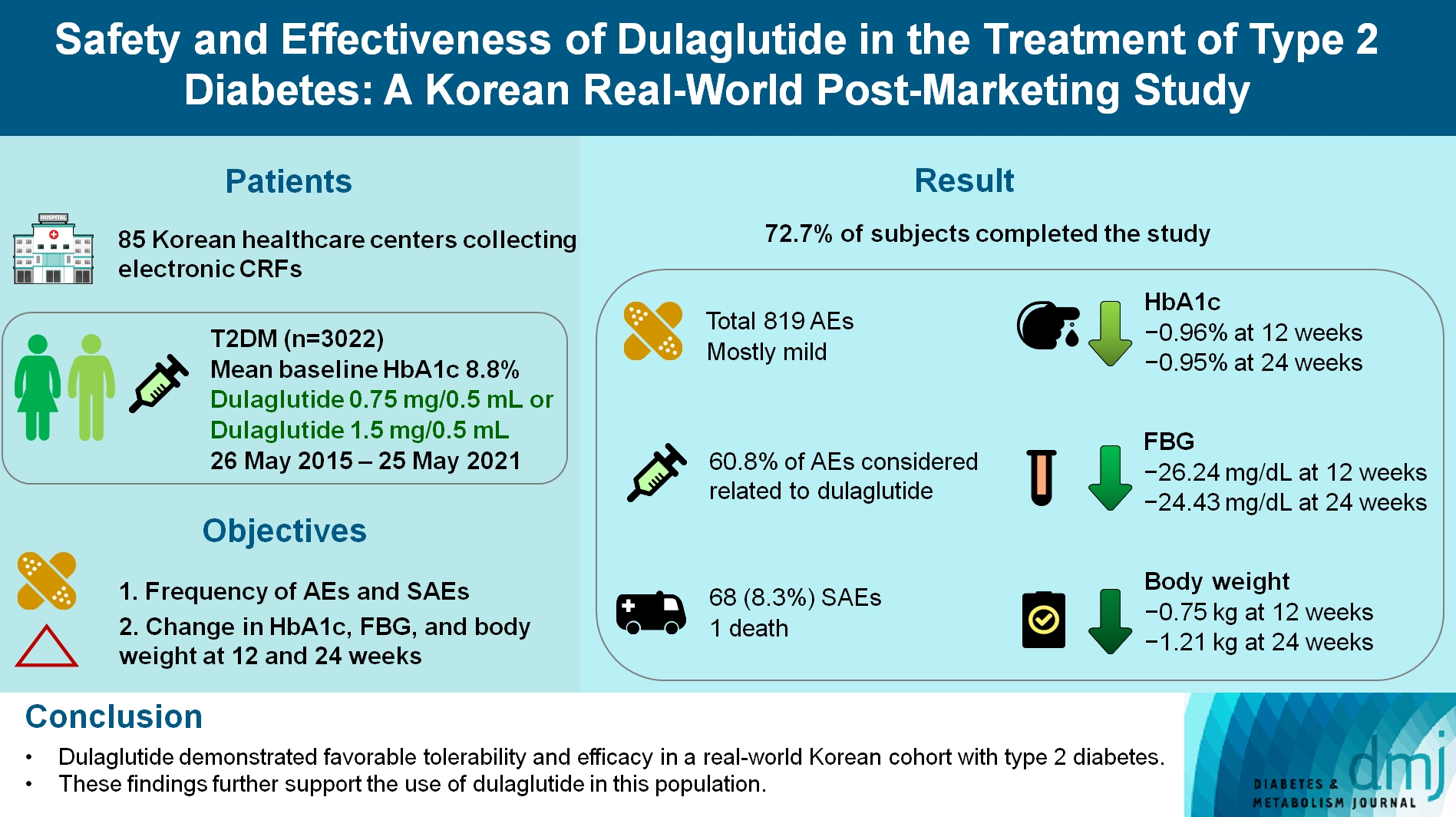
To investigate the real-world safety and effectiveness of dulaglutide in Korean adults with type 2 diabetes mellitus (T2DM).
Methods
This was a real-world, prospective, non-interventional post-marketing safety study conducted from May 26, 2015 to May 25, 2021 at 85 Korean healthcare centers using electronic case data. Data on patients using dulaglutide 0.75 mg/0.5 mL or the dulaglutide 1.5 mg/0.5 mL single-use pens were collected and pooled. The primary objective was to report the frequency and proportion of adverse and serious adverse events that occurred. The secondary objective was to monitor the effectiveness of dulaglutide at 12 and 24 weeks by evaluating changes in glycosylated hemoglobin (HbA1c ), fasting plasma glucose, and body weight.
Results
Data were collected from 3,067 subjects, and 3,022 subjects who received ≥1 dose (of any strength) of dulaglutide were included in the safety analysis set (53% female, mean age 56 years; diabetes duration 11.2 years, mean HbA1c 8.8%). The number of adverse events reported was 819; of these, 68 (8.3%) were serious adverse events. One death was reported. Adverse events were mostly mild in severity; 60.81% of adverse events were considered related to dulaglutide. This study was completed by 72.73% (2,198/3,022) of subjects. At 12/24 weeks there were significant (P<0.0001) reductions from baseline in least-squares mean HbA1c (0.96%/0.95%), fasting blood glucose (26.24/24.43 mg/dL), and body weight (0.75/1.21 kg).
Conclusion
Dulaglutide was generally well tolerated and effective in real-world Korean individuals with T2DM. The results from this study contribute to the body of evidence for dulaglutide use in this population.

 KDA
KDA First
First Prev
Prev





